Way too many developments in the past month, at least half of which probably deserved their own post.
Wounding, Interleukin-1 (IL-1) and γδ T-cells
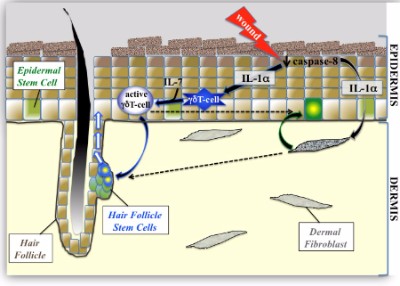
At the end of 2013. I first discussed Dr. Krzysztof Kobielak and Dr. Eve Kandyba from USC in relation to their work on how the Wnt7 gene activates hair growth. Now, both scientists are back in the news and have collaborated with researchers from UC San Diego and India and published a paper titled: “Stimulation of hair follicle stem cell proliferation through an IL-1 dependent activation of γδT-cells“. Interestingly, one of the three most important word used throughout the paper (“wounding” or “wound”) is missing from that title, although not from the complementing photo (see red text in the image further below).
The scientists have found that wounding triggers interleukin-1 (IL-1), which in turn activates certain immune cells (called γδT-cells or gamma delta cells). These immune cells then awaken resting stem cells in the hair follicle (so that they can then “multiply and travel to the wound site to repair the injury”). For our purposes, the hair follicle stem cell activation is more important than any kind of wound repair. Hair is obviously more important than scar repair or infection prevention:-)
Commentator “Omg” who has posted some great material in recent weeks (including the above — thanks!) wrote that this finding is the biggest discovery of the past decade (I am not so sure). Note that the authors of this latest work are not affiliated with Follica (the widely discussed company that is developing a “wounding+favorable compound injection” related procedure to regrow hair); nor are they affiliated with Dr. Rachita Dhurat, who has published several groundbreaking papers related to wounding and hair growth. Makes this discovery from a different group all the more exciting, and further validates the potential of wounding led hair regrowth.
![Interleukin-1 and hair growth]()
Nanogel Encapsulated Dermal Papilla 3D Spheroids and Hair Follicle Regeneration
In 2015 I interviewed Dr. Malcolm Xing from Canada. In 2016, I discussed the same doctor’s new findings related to a novel improved “hanging drop” 3D cell culturing technique for the purpose of hair growth. Dr. Xing has constantly been trying to improve upon the renowned Dr. Colin Jahoda’s work.
This month, Dr. Xing and a team from China have published a new paper titled “Bottom-up Nanoencapsulation from Single Cells to Tunable and Scalable Cellular Spheroids for Hair Follicle Regeneration“. I am not motivated enough to try to make the sci-hub site work (I get mixed luck with that) to gain free access to this whole paper, since I do not want to then spend hours trying to understand the complicated science behind this research. I am, however, in no doubt whatsoever that any new development related to 3D spheroids and hair is of utmost importance and warrants coverage.
More Good News from Aclaris
In November, I discovered an interview in which Aclaris Therapeutics’ chief scientific officer mentioned that in the first half of 2018, Aclaris planned to commence phase 2 trials to treat androgenetic alopecia (male pattern hair loss) with topical JAK inhibitors. This was a huge development, since we are used to ALL companies in the hair loss world postponing or delaying clinical trials. In contrast, Aclaris is doing the opposite and moving to phase 2 trials without even having done phase 1 trials! They probably got to skip phase 1 trials because they have tested the same JAK inhibitors (including oral and topical) for other conditions.
Several weeks after I posted the above pleasing development, Aclaris announced at an investor conference that they would start the above trials at the end of the first quarter of next year (i.e, end of March 2018). Yet again they surprise us with their speed, and do not want to wait will June 2018. The announcement can be accessed by going to their website and finding their latest investor conference presentations (often available in both audio and slide formats). Thanks to “Royaume” who listened to the whole audio for us (albeit misinterpreted its implication :-)). Commentator “Malcolm” even felt like I should have devoted a whole post to this news, but I did not want to do so less than a month after my prior post on Aclaris. Moreover, past experiences make me think that Aclaris will delay its trial commencement target dates (like all others who came before it seem to do), although I hope I am wrong.
And as if this was not enough, earlier today it was announced that Aclaris has received its first ever product approval by the FDA for the former’s ESKATA hydrogen peroxide topical solution (to treatment raised seborrheic keratoses). Funnily enough, Aclaris stock declined today despite this widely publicized good news. Thank goodness I never pursued my dream of part-time day trading.
Follicum also Plans Phase 2 Trials in Early 2018
Even though Follicum has been present in the hair loss world well before Aclaris even commenced operations, the former has not moved along as fast as the latter. However, recently Follicum announced that they were planning to start phase 2 trials for their FOL-005 product in early 2018 in Germany.
Replicel CEO Update
Replicel’s CEO was interviewed by “Investing News” towards the end of last month. Key quote:
“There’s a possibility… that [our] hair products could have an early launch in Japan. Our partner Shiseido is funding a clinical trial in Japan that’s expected to release clinical data next year. It’s entirely up to Shiseido what they do in regards to this product. There’s certainly a possibility that they could decide if the data is positive, to launch the product in Japan and that would trigger… milestone payments and sales royalty revenue”.
Other Items of Interest
— I saw a number of comments and received several e-mails regarding the Brotzu lotion. It seems like they updated their EU patent and there are Italian hair loss forum rumors that the product is coming out in 2018. I might update with better info, but for the time being it seems like a lot of speculation going on.
— A great thread with before and after photos on Reddit about the power of the “Big 3” treatment of Finasteride plus Minoxidil plus Nizoral when effective.
— PolatiyTE starts using its SkinTE product on humans. No info yet on potential hair growth effects on the scalp, but still great to see usage in humans rather than mice so early in the process. Key quote:
“We are confident and believe that SkinTE will replicate its preclinical success and help patients regenerate their own full-thickness, hair-bearing skin”.